medical and scientific
We work with clients to develop medical devices and scientific instruments across a broad range of speciality areas.
We provide clients needing to scale-up in the medical and scientific sector with the technical and development support they need.

outerspace understands the medical product development process and the unique language, standards and guidelines of regulated medical device development. We work closely with regulatory and quality specialists to develop appropriate strategies. With years of experience in Class 1 and Class 2 medical product development, we translate and help verify our client’s core technologies into viable manufacturable products.
We understand patients, conditions and the environments that diagnostic devices and instruments are used. We work with clinical specialists to improve tools and processes to improve clinical outcomes.
We have years of experience in a wide range of medtech diagnostic sectors ranging from neurology and opthalmics; to cardiac instruments, in vitro devices, hearing device systems, drug delivery products and wound treatment devices.
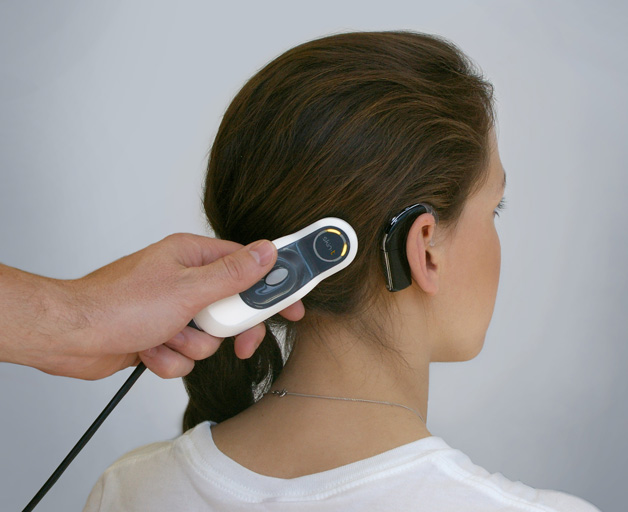

In the complex neurological device sector we design devices and systems to treat epilepsy; sleep monitoring systems to detect sleep disorders and prevent sleep apnoea; and devices used to treat nerve damage.
We are an integral partner in ophthalmic instrument and device development. We’ve developed tonometers, corneal topographic imaging systems and surgical tools to guide ophthalmic surgery.
related work
our experts
explore insights and ideas
stay up to date with product development trends, our latest projects and news








